PFAS
Chromatographic Specialties is your all-in-one resource for PFAS consumables, technical help, new developments, and instruments!
One purchase order, one invoice, one shipment — save yourself time, money and hassles!
Our offering continues to grow as we solidify our place as THE source of PFAS products in Canada.
Suppliers

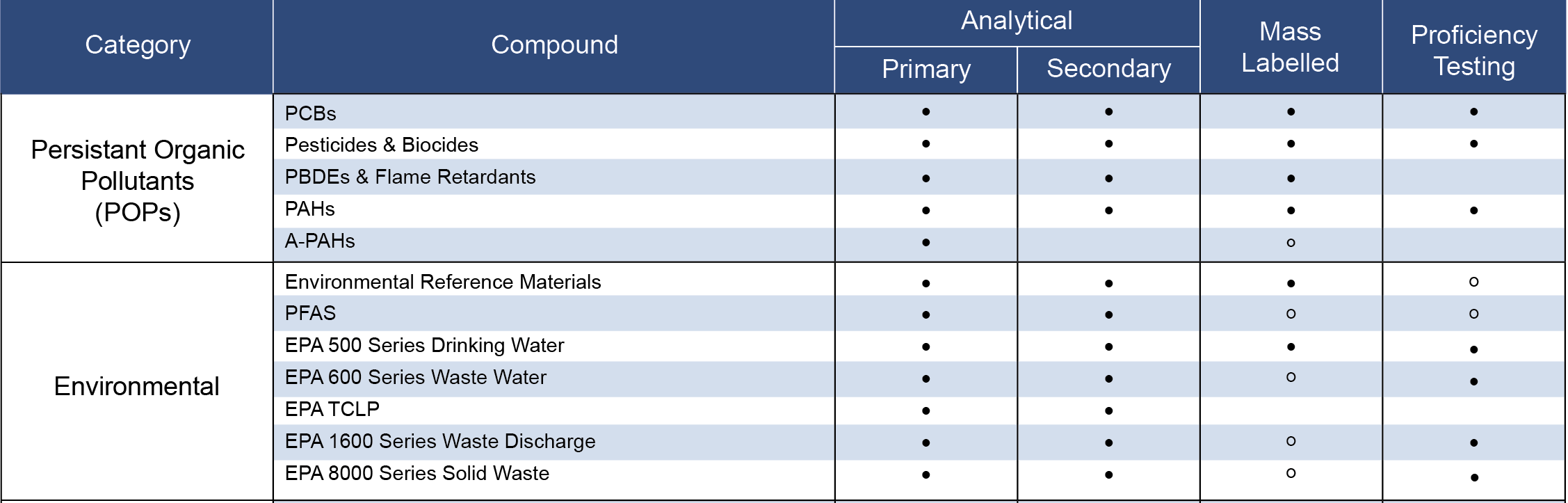
PFAS Products
PFAS Reference Materials
• Accustandard
• Restek
• LGC
Columns
• Restek Raptor & Force LC Columns
• Delay Columns
Sample Preparation
• Ultra-clean Resin for Air Analysis
• ASE Cells & Parts
• Filters
• QuEChERS
• SPE
Caps and Vials
Instruments
PFAS Application Notes
Analysis of PFAS in Aqueous Samples according to EPA Draft Method 1633
In September 2021, the United States Environmental Protection Agency (US EPA) published a draft method for the analysis of per- and polyfluoroalkyl substances (PFAS). The draft method is a single laboratory validated method to test for 40 PFAS compounds in a diverse range of environmental matrices including wastewater, surface water, groundwater, soil, biosolids, sediment, landfill leachate, and fish tissue.The guideline can be used in various applications, exemplarily for use in the Clean Water Act (CWA) or the National Pollutant Discharge Elimination System (NPES)Application benefitsSuccessful determination of 40 Per- and Polyfluoroalkyl Substances from water samples according to EPA Draft Method 1633High recovery rates were achieved with a CHROMABOND® WAX SPE columnFast and sensitive HPLC analysis on a NUCLEODUR® PFAS column
Eliminate the Impact of Instrument-Related PFAS Interferences by Using a Delay Column
Poly- and perfluoroalkyl substances, or PFAS, are rapidly emerging as some of the most important environmental contaminants to monitor around the world. Their widespread use and environmental persistence make them truly a global issue. Concerns over possible health risks are driving environmental...
This application note outlines a simple SPE procedure for the extraction of 26 diverse per- and polyfluoroalkyl substances (PFASs) in drinking water using UCT’s polymeric weak-anion exchange SPE cartridges (ENVIRO-CLEAN® WAX)...
Analysis of Ultrashort-Chain and Short-Chain (C1 to C4) PFAS in Water Samples
Due to their ubiquitous occurrence in aquatic environments, measuring ultrashort-chain per- and polyfluoroalkyl substances (PFAS) in water to monitor their presence and the potential for human exposure has become very important. With carbon chain lengths less than C4, these small, highly polar compounds are difficult to analyze using reversed-phased liquid chromatography (RPLC). In this study, an accurate, reliable analytical LC-MS/MS method for PFAS in water was developed to specifically quantify C1 to C4 PFAS in both potable and non-potable sources. A direct injection workflow was implemented to simplify the testing process and to avoid potential contamination originating from poor sample preparation procedures
C1-C10 PFAS Analysis in Human Plasma and Serum
Monitoring ultrashort-chain (C1 to C3) PFAS is essential for tracking human exposure and health consequences, but a lack of analytical methods has limited testing efforts. This new LC-MS/MS method allows testing of C1 to C10 carboxylic and sulfonic acid PFAS, along with four alternative compounds, in human plasma and serum.
Fast Fluorotelomer Alcohol Analysis Method
Fluorotelomer alcohols (FTOHs) have recently emerged as an important class of PFAS compounds in environmental testing laboratories. These compounds are commonly used in manufacturing materials for their oil and water-repellant capabilities but have recently been found to degrade into toxic compounds that pose health and environmental concerns. The degradation products of these volatile FTOHs are frequently detected in indoor and outdoor air samples and thus are important to monitor. Since FTOHs are an emerging class of compounds, testing methodologies are limited. Restek has developed a rapid analysis of four common fluorotelomer alcohols on the LPGC Rtx-200,achieving excellent separations with a run time of under four minutes.
Technical Tips
Restek – PFAS HPLC Column Anatomy:
Which Phase, Dimensions, and Particle Types are Best?
As interest grows, the need for fast, accurate, and precise testing is expanding with it. This demand is driving the development of better methods, and LC column selection is the foundation for building an improved approach. This becomes particularly important as the list of monitored PFAS grows to include shorter and shorter alkyl chain compounds. Here, we’ll examine the properties that are important to consider when choosing an LC column for PFAS analysis.
Silcotek – Non-PFAS Coating Alternatives
Restek – How do I know if my LC system has PFAS contamination?
The easiest way to identify PFAS contamination in your LC is to pump mobile phase through the column for 30 minutes to give system-related PFAS a chance to build up at the head of the column. Then, inject a solvent blank, and when the gradient runs through the column, any PFAS that built up on the analytical column will elute and appear at the retention time matching where PFAS in your sample would elute.
Next, inject another solvent blank immediately after the solvent blank injection with the long equilibration time and look at the retention times for PFAS compounds. The purpose of this second injection is to not give enough time for the system-related PFAS to build up in your analytical LC column.
Compare the results of the immediate injection to those of the long equilibration time injection. If there are PFAS peaks in the long equilibration time injection, but not in the injection that immediately followed it, your LC contains system-generated PFAS contaminants that could interfere with trace analysis. If there are not any peaks at the retention times of the PFAS you are looking for in both injections, your instrument may not have a significant amount of system-related PFAS interference. This can occur if you are using an old LC unit with a new mass spectrometry system because most of the leachable, system-related PFAS have already leached out. However, this is not a permanent state; if you replace plastic components in any part of the instrument before the injector, it is highly likely that system-related PFAS interference will leach out unless the new parts are PFAS-free.
Restek – Why are system-related PFAS important to isolate from my samples?
If you are analyzing PFAS at very low levels, like parts per trillion (ppt), for accurate identification and quantitation, you need to make sure that the PFAS detected belong only to your sample. If background PFAS contamination from your LC coelutes with sample PFAS, they will interfere and cause inaccurate results.
Restek – How does the PFAS delay column help?
A PFAS delay column is installed before the injector, so it traps system-related PFAS and delays their elution. This prevents them from coeluting and interfering with the PFAS in the sample. Functionally, as the gradient starts, the trapped PFAS are eluted from the delay column and then travel to the analytical column. They arrive at the analytical column after the PFAS from the injected samples, so they do not coelute with the sample PFAS.
PFAS in the sample will elute as a normal-shaped, symmetrical peak but the delayed system-related PFAS have been continuously moving throughout the system with the gradient. They were never focused on the analytical column, so when they elute, the “peak” they generate is just an elevated baseline. You know it belongs to one or more PFAS because the signal matches the MS/MS transition of the compounds you are monitoring. However, because it is after the retention time window of the same PFAS in your sample, it’s not detected and quantitated as part of the sample.
Restek – What’s different about PFAS analysis compared to other LC-MS/MS analyses?
LC-MS/MS analysis is a very sensitive, selective technique that is used in many different applications and is ideally suited for multi-analyte analysis. Due to their chemical/physical properties and long-lasting inertness, plastic fluoropolymers, such as polytetrafluoroethylene (PTFE), are used to make many LC-MS/MS components. You can find PTFE in LC pump seals, mobile phase transfer lines, the plastic linings used in degassers, etc. Unfortunately, these plastic parts can leach PFAS into the mobile phase, which creates system-related background PFAS contamination that interferes with trace-level PFAS analysis. Because PFAS detection levels in drinking water are in ppt range, even slight interference can bias quantitative analysis.
PFAS interference can also come from the solvents (even new bottles) used to make mobile phase due to the ubiquitous use of PFAS compounds. PFAS are found in the air as well, so you can assume PFAS contaminants are literally everywhere at low concentration. Basically, system-related PFAS come from components in all stages of the LC-MS/MS workflow, including the sample collection bottle; mobile phase cap/lining (made of PTFE); solvent inlet tubing (often FEP or PFA); degasser; LC pump parts; and even the autosampler vial septum (many are PTFE-lined silicone).
The degree of interference that is observed will vary across LC instrument manufacturers and also depends on method parameters (e.g., equilibration time, solvent choice, target analytes, etc.) Note that the longer equilibration times produce more interference because PFAS have more time to leach out of the plastic parts.
Restek – What are typical PFAS analysis levels?
PFAS detection levels vary by region and across matrices such as drinking water, wastewater, seawater, soil, etc. Drinking water is the matrix of most concern in relation to public health and many environmental agencies are trying to set safe consumption levels. Most are in the form of health-based recommended levels with no regulations in place as of Feb 2019. Currently, in most countries, designated levels of concern are in the low ppt (ng/L) range for most PFAS compounds in drinking water. Some examples are given below.
• United States: health advisory level of 70 ppt in drinking water for PFOS and PFOA combined
• Sweden: action level of 90 ppt in drinking water for 11 PFAS compounds combined
• Italy (Veneto): threshold values in drinking water, PFOS ≤ 0.03 ppt, PFOA ≤ 0.5 ppt, other PFAS ≤ 0.5 ppt
• Germany: health-based precautionary value of 100 ppt in drinking water for PFOA, PFOS, and many other PFAS
• Australia: 70 ppt for the sum of PFOS and PFHxS and 560 ppt for PFOA in drinking water with different levels for other types of waters
LCGC – A Review of the Latest Separation Science Research in PFAS Analysis
LCGC – Characterizing a Mixed Mode Fluorocarbon/Weak Anion Exchange Sorbent for PFAS Separation
Credit:
(14) Abdelraheem, E.; Wise, J.; Murphy, C. et al. Triple-Stage Quadrupole Mass Spectrometer to Determine Ubiquitously Present Per- and Polyfluorinated Alkyl Substances in Drinking Water at Part Per Trillion Levels Using Solid Phase Extraction Approach. Bull. Environ. Contam. Toxicol. 2023, 110, 32. DOI: 10.1007/s00128-022-03686-1
Cheryl Murphy is now working in Michigan, but her undergrad at Dal and masters at UofA.
Literature








